Key Highlights:
- Phase 1/2 safety cohort enrollment complete: Eight (8) patients with invasive oral cavity cancer treated in the PRV211 safety cohort
- Primary safety endpoint met: Safety assessed by clinical laboratory evaluations and/or clinical examination at 1-month post-surgery
- Favorable safety profile observed: No treatment-related serious adverse events, dose-limiting toxicities, or systemic toxicities reported
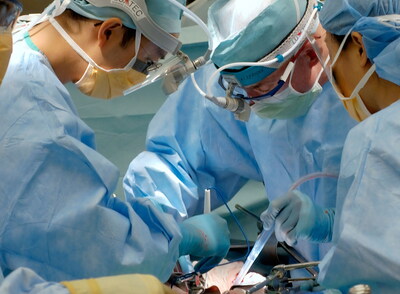
BOSTON, Jan. 23, 2026 /PRNewswire/ — Privo Technologies, Inc., (“Privo”) today announced that enrollment has been completed for Arm 2 of the CLN-004 clinical trial evaluating PRV211, and that the study met its primary safety endpoint. The primary objective of this study arm was to evaluate the safety profile of PRV211 treatment, with safety assessed based on clinical laboratory evaluations and/or clinical examination conducted at one month following surgery.
PRV211 builds on the nanoengineered polymer platform of PRV111, and is designed as a sterile intraoperative patch intended for integration into standard surgical workflows. PRV211 was administered intraoperatively immediately following tumor resection, with the objective of delivering localized chemotherapy to the surgical bed while minimizing systemic exposure. The overall development objective of PRV211 is to reduce recurrence risk by applying localized therapy to the tumor bed at the time of surgery. Post-surgical recurrence remains a significant unmet clinical challenge in oral cavity cancer. To date, PRV211 has demonstrated a favorable safety profile consistent with expectations for Privo’s localized delivery platform.
Safety Outcomes and Follow-Up
The Phase 1/2 safety cohort enrolled eight (8) patients diagnosed with invasive oral cavity cancer requiring surgical excision. Follow-up assessments conducted at protocol-specified visits demonstrated:
- No treatment-related serious adverse events (SAEs)
- No systemic toxicities
- No dose-limiting toxicities (DLTs)
- No reports of delayed wound healing or healing complications
PRV211 was well tolerated based on clinical laboratory assessments and post-surgical examinations conducted at the one-month follow-up visit. PRV211 pharmacokinetic profile assessments support negligible systemic platinum absorption, validating targeted delivery with minimal off-target effects. In addition to safety follow-up, all eight subjects will continue to be monitored for efficacy outcomes, defined as assessment of loco-regional recurrence at 12 months post-surgery.
“Completion of enrollment and achievement of the primary safety endpoint represent important milestones in the clinical development of PRV211,” said Dr. Manijeh Goldberg, PhD, Founder and CEO of Privo Technologies. “The ongoing follow-up of these patients for loco-regional recurrence will help inform the design of future studies as we continue to evaluate PRV211’s potential role as a localized, intraoperative treatment approach.”
Ongoing CLN-004 Clinical Program
PRV211 represents Arm 2 of Privo’s multi-asset CLN-004 clinical program, which is evaluating localized therapeutic approaches for oral cavity cancers. Arm 2 is focused on establishing the safety and feasibility of intraoperative PRV211 administration. A future expansion study is planned to further evaluate the efficacy of PRV211 based on loco-regional recurrence outcomes.
With the completion of Arm 2 enrollment, in addition to the primary efficacy endpoint being met in Arm 1 and the successful dosing initiation of Arm 3, the CLN-004 program continues to advance in accordance with its clinical development plan, supporting Privo’s broader portfolio of localized therapies for oral cavity malignancies.
About Privo Technologies, Inc.
Privo Technologies is a late-stage clinical biopharmaceutical company pioneering localized, minimally invasive therapies for solid tumors using its proprietary nanoengineered delivery platform. Privo is committed to developing innovative, patient-centric therapies that improve outcomes and preserve quality of life for patients with solid tumors.
Learn more at www.privotechnologies.com
For media inquiries: [email protected]
For business inquiries: [email protected]
View original content to download multimedia:https://www.prnewswire.com/news-releases/privo-technologies-announces-milestone-in-prv211-arm-of-cln-004-clinical-trial-primary-endpoint-met-no-serious-adverse-events-reported-302668552.html
SOURCE Privo Technologies