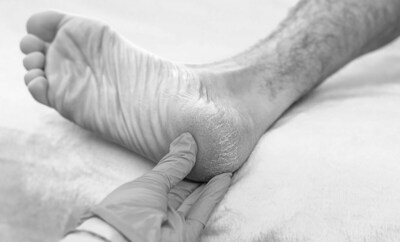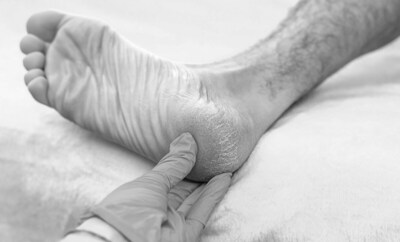
PENSACOLA, Fla., Feb. 4, 2026 /PRNewswire/ — Regenative Labs, in collaboration with Babak Baravarian, DPM, Director of Foot & Ankle Research at University Foot & Ankle Institute, today announced the publication of a new observational case series examining the use of Wharton’s jelly connective tissue allografts in patients with plantar fascia defects associated to plantar fasciopathy.
The study, titled “Wharton’s Jelly Tissue Allografts for Tearing in the Plantar Fascia: A Case Series,” was published on September 24, 2025, in Biomedicines, a peer-reviewed, open-access medical journal. The case series reports outcomes from nine patients with plantar fasciopathy based defects who received a single ultrasound-guided application of a collagen-rich Wharton’s jelly connective tissue allograft and were followed for 90 days.
Plantar fasciopathy—commonly referred to as plantar fasciitis—characterized by microtearing of the plantar fascia that can significantly impact mobility and quality of life.
“The single best method we’ve ever used in the care of plantar fasciopathy has been Wharton’s jelly umbilical cord tissue allografts,” said Babak Baravarian, DPM, senior author of the study and Director of Foot & Ankle Research at University Foot & Ankle Institute.
Study Findings
In this small observational cohort (n=9; mean age 73), patients demonstrated an overall trend toward improvement across pain, function, and quality-of-life measures over the 90-day follow-up period. Reported outcomes included:
- 60.98% mean improvement in Numeric Pain Rating Scale (NPRS) scores from baseline to Day 90
- 49.55% mean improvement in WOMAC Total scores from baseline to Day 90, with statistically significant improvements observed across total and subscale measures
- No adverse reactions reported during the observational period
“These findings contribute to a growing body of peer-reviewed literature examining Wharton’s jelly in homologous musculoskeletal applications,” said Naomi Lambert, corresponding author and Research Scientist at Regenative Labs. “They also highlight the importance of structured outcome tracking in regenerative medicine research.”
The authors note limitations typical of early-stage observational research, including a small sample size, the absence of a control group, and reliance on patient-reported outcomes. The study recommends further prospective research to evaluate safety, consistency, and clinical potential in larger populations.
Context and Clinical Relevance
The authors frame plantar fasciopathy as a degenerative, collagen-based condition rather than a purely inflammatory process. This perspective supports emerging approaches in conservative musculoskeletal care that focus on tissue maintenance and structural support, guided by diagnostic imaging and longitudinal outcome tracking.
Study Overview
- Design: Observational case series with follow-ups at baseline, 30 days, and 90 days
- Population: 9 patients with plantar fasciopathy (mean age 73; 5 male, 4 female)
- Eligibility: Patients had failed at least three months of conservative therapy prior to allograft application
- Intervention: Single ultrasound-guided application of 2 cc of 150 mg/mL Wharton’s jelly connective tissue allograft into areas of confirmed degeneration
- Outcomes Measured: NPRS, WOMAC, and Quality-of-Life Scale (QOLS)
- Safety: No adverse reactions reported
“This publication reflects our commitment to clinician-driven, IRB-backed research that emphasizes safety, transparency, and responsible data collection,” said Tyler Barrett, CEO of Regenative Labs and a co-author of the study. “Our role is to support physicians with compliant tissue processing and research infrastructure so meaningful clinical questions can be evaluated rigorously.”
Transparency and Disclosure
The authors disclose relationships with Regenative Labs, including company involvement in aspects of study design, analysis, manuscript preparation, and the decision to publish. The study reports institutional review board (IRB) oversight and informed consent.
About the Publication
Biomedicines is an international, peer-reviewed, open-access journal published by MDPI. The full article is available online.
About Wharton’s Jelly Connective Tissue Allografts
Wharton’s jelly is an umbilical cord–derived connective tissue rich in extracellular matrix components. The publication discusses its structural composition and use as a homologous tissue allograft in musculoskeletal applications.
About University Foot & Ankle Institute
University Foot & Ankle Institute is a nationally recognized podiatric medical group specializing in complex foot and ankle pathology, biomechanics, and advanced non-surgical and surgical interventions, with an active focus on clinical research.
About Regenative Labs
Regenative Labs is a Pensacola, Florida–based tissue processing organization. The study notes that Regenative Labs follows FDA- and American Association of Tissue Banks (AATB)-aligned tissue processing protocols as described by the authors.
Important Information
This press release describes findings from a small observational case series. Observational research results may not be generalizable to all patients. This material is for informational purposes only and does not constitute medical advice. Patients should consult a qualified healthcare professional regarding diagnosis and treatment options.
Media Contact
Evan Dempsey
Regenative Labs
Phone: +1-800-891-3452
Email: [email protected]
Scientific / Publication Contact
Naomi Lambert
Regenative Labs
Phone: +1-800-891-3452
Email: [email protected]
View original content to download multimedia:https://www.prnewswire.com/news-releases/new-published-case-series-explores-umbilical-cord-tissue-allografts-in-plantar-fascia-care-302679620.html
SOURCE Regenative Labs