New approval brings most advanced, efficient Exablate platform to Canada, expanding access to enhanced care for people living with essential tremor
HAIFA, Israel and MIAMI, Feb. 5, 2026 /PRNewswire/ — Insightec, a global healthcare company dedicated to using focused ultrasound to transform patient care, today announced it has received a Medical Device License from Health Canada for its Exablate® Prime system. Previously approved by the U.S. Food and Drug Administration (FDA), this approval expands access to incisionless, MR-guided focused ultrasound treatments, providing relief for patients in Canada living with essential tremor.
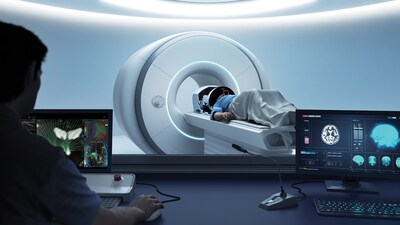
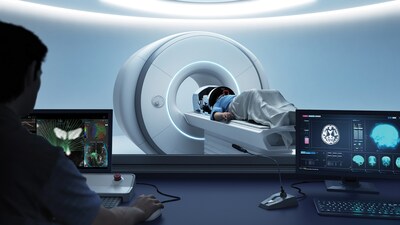
Exablate Prime builds on the success of its predecessor and has demonstrated shorter procedure times with efficient workflows and hardware. The system features a more intuitive user interface with guided workflows and automated steps that simplify planning. Intelligent algorithms and refined visualization options provide enhanced insights that may better inform clinical decision making. Additional upgrades, including improved cybersecurity and remote planning, further support a smoother, more productive clinical workflow. This approval also expands MR system compatibility. Exablate Prime is now available for use with select Philips, in addition to GE and Siemens, MR systems at treatment centers throughout Canada.
“This Medical Device License from Health Canada marks an important step in expanding access to advanced focused ultrasound treatment for people living with Parkinson’s disease and essential tremor in Canada,” said Maurice R. Ferré, MD, CEO and Chairman of Insightec. “By bringing Exablate Prime to more treatment centers, we aim to support clinicians with an enhanced platform and help more patients access incisionless treatment options today, while continuing to advance focused ultrasound for the future.”
Focused ultrasound is delivered in a single, outpatient procedure, with many patients experiencing immediate improvement and minimal or no complications. To date, more than 30,000 patients have been treated with Insightec’s Exablate technology for a variety of movement disorder indications at 214 centers worldwide.
About Insightec
Insightec is a global healthcare company creating the next generation of patient care by realizing the therapeutic power of acoustic energy. The company’s Exablate Neuro platform focuses sound waves, safely guided by MRI, to provide treatment to patients with medication-refractory essential tremor and Parkinson’s disease*. Research for future applications in the neuroscience space is underway in partnership with leading academic and medical institutions. Insightec is headquartered in Haifa, Israel and Miami, with offices in Dallas, Texas; Frankfurt, Germany; Shanghai; and Tokyo.
Follow us on LinkedIn, Facebook, Instagram, YouTube and X or visit insightec.com for more information. For detailed country specific indications visit https://insightec.com/regulatory-approvals/.
Forward-looking Statements:
This document contains forward-looking statements regarding, among other things, plans, expectations and future events. In some cases, forward-looking statements can be identified by the following words: “may,” “can,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “promise,” “continue,” “ongoing,” or the negative of these terms. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. Any forward-looking statement is based on information available to Insightec as of the date of the statement. All written or oral forward-looking statements attributable to Insightec are qualified by this caution. Insightec does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Insightec’s expectations.
“Exablate,” and “Exablate Neuro,” as well as the “INSIGHTEC” logo, whether standing alone or in connection with the word “Insightec,” are protected trademarks of Insightec.
Insightec Media Contact:
G&S Business Communications for Insightec
Marjani Williams
[email protected]
(312) 648-6700, ext.2108
Photo – https://mma.prnewswire.com/media/2876908/Insightec_Exablate_Prime_Console.jpg
Logo – https://mma.prnewswire.com/media/1699588/5752090/Insightec_Logo.jpg
View original content to download multimedia:https://www.prnewswire.com/news-releases/insightec-secures-health-canada-approval-for-exablate-prime-system-302680158.html
SOURCE Insightec