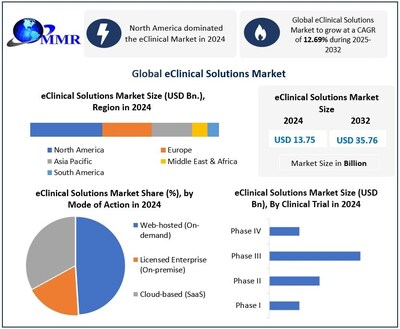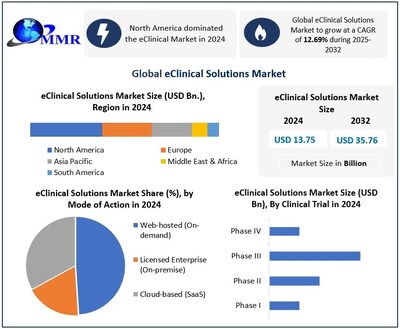
PUNE, India, Feb. 10, 2026 /PRNewswire/ — Global eClinical Solutions Market size was valued at USD 13.75 billion in 2024 and is projected to grow at a CAGR of 12.69% from 2025 to 2032, reaching nearly USD 35.76 billion by 2032.
eClinical Solutions Market is set for robust growth, driven by the adoption of AI-powered platforms, cloud-based clinical trial management, and decentralized trial models. Increasing demand for patient-centric solutions, real-time data analytics, and integrated digital trial ecosystems is fueling widespread industry expansion.
Get Full PDF Free Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @ https://www.maximizemarketresearch.com/request-sample/3843/
“Decentralized trials, AI, and cloud platforms are transforming clinical research; explore the future of eClinical Solutions with Maximize Market Research!”
Key Market Trends & Insights from the eClinical Solutions Market Report
- Based on product type, Clinical Trial Management Systems (CTMS) captured the leading share in 2024, driven by their role as the operational backbone of modern trials. Sponsors and CROs are increasingly deploying integrated CTMS platforms to manage study planning, site performance, budgeting, and real-time trial tracking across global, multi-site programs.
- Electronic Data Capture (EDC) platforms remain the foundational technology in the eClinical Solutions Market, serving as the primary engine for structured clinical data collection. However, the segment is evolving beyond traditional form-based capture toward unified clinical data platforms that integrate real-world data, imaging, genomics, and wearable-generated endpoints.
- Cloud-based clinical trial management solutions are rapidly replacing on-premise deployments, particularly among mid-size biopharma companies and global CRO networks. The shift toward Software-as-a-Service (SaaS) eClinical platforms is enabling faster study start-up, remote collaboration, and scalable infrastructure across decentralized clinical trial environments.
- Decentralized clinical trial software is emerging as a strategic growth engine, with sponsors increasingly adopting remote monitoring, telehealth integrations, and digital patient engagement tools. Hybrid and fully decentralized trial models are expected to become standard in late-phase and post-approval studies by the end of the forecast period.
- AI-powered eClinical platforms are redefining trial execution and decision-making, enabling predictive patient matching, automated risk-based monitoring, and real-time safety analytics. These capabilities are expected to shorten enrollment cycles, reduce protocol deviations, and improve overall trial success rates.
- eCOA and digital patient-reported outcome tools are gaining strong adoption, particularly in late-phase and patient-centric studies. Their ability to collect high-frequency, real-world patient data is improving endpoint accuracy, retention rates, and regulatory acceptance.
- Pharmaceutical and biopharmaceutical companies remained the dominant end-user segment in 2024, accounting for the majority of eClinical platform investments. These organizations are increasingly adopting integrated eClinical software platforms to accelerate drug development timelines and support complex, multi-arm trials.
- North America accounted for the largest share of the global eClinical Solutions Market in 2024, supported by high clinical trial activity, strong regulatory frameworks, and advanced digital infrastructure. The region continues to lead in the adoption of AI-driven clinical trial management solutions and unified data platforms.
- The competitive landscape is increasingly defined by end-to-end platform providers, with major players focusing on integrated clinical, regulatory, and quality ecosystems. Strategic partnerships, AI integration, and unified development clouds are becoming key differentiators among leading vendors.
Cloud, AI, and CTMS: The Segments Powering the Next Wave of the eClinical Solutions Market
eClinical Solutions Market is entering a new phase where CTMS and EDC market trends are converging with cloud-based clinical trial management solutions to create unified digital trial ecosystems. As sponsors accelerate adoption of AI-powered eClinical platforms and decentralized clinical trial software, segmentation is shifting toward scalable, integrated architectures. This transition is redefining how pharmaceutical companies and CROs deploy digital clinical trial solutions to improve speed, accuracy, and global trial coordination.
Get Insightful Data on Regions, Market Segments, Customer Landscape, and Top Companies (Charts, Tables, Figures and More) – https://www.maximizemarketresearch.com/request-sample/3843/
By Product
Clinical Trial Management Systems (CTMS)
Electronic Clinical Outcome Assessment (eCOA)
Electronic Data Capture (EDC)
Clinical Data Management Systems (CDMS)
Electronic Trial Master Files (eTMF)
Randomisation and Trial Supply Management (RTMS)
Safety Solutions
Others
By Mode of Delivery
Web-hosted (On-demand)
Licensed Enterprise (On-premise)
Cloud-based (SaaS)
By Clinical Trial
Phase I
Phase II
Phase III
Phase IV
By End-User
Pharmaceuticals & Biopharmaceutical Companies
Contract Research Organizers
Hospitals
Medical Device Manufacturer
Consultancy Service Companies
Academic & Research Institutes
Scope of the report includes below the solutions:
Data Capture & Management Solutions
• Electronic Data Capture (EDC)
• Clinical Data Management Systems (CDMS)
• eSource solutions
Clinical Trial Operations Solutions
• Clinical Trial Management Systems (CTMS)
• Randomization and Trial Supply Management (RTSM/RTMS)
• Electronic Trial Master File (eTMF)
Patient-Centric & Outcome Assessment Solutions
• Electronic Clinical Outcome Assessment (eCOA/ePRO/eClinRO)
• eConsent solutions
• Decentralized/virtual trial platforms
Analytics & Integration Solutions
• Clinical analytics and reporting platforms
• Risk-based monitoring (RBM) tools
• Clinical data integration platforms
Safety & Compliance Solutions
• Pharmacovigilance and safety solutions
• Regulatory and quality management tools
Some of the emerging platforms and technologies in the pipeline include
• AI-enabled clinical trial platforms
• Predictive patient recruitment and site selection tools
• Digital biomarkers and wearable-integrated data capture
• Real-time analytics and synthetic control arm solutions
• End-to-end unified eClinical suites
• Remote monitoring and decentralized trial technologies
Immediate Delivery Available | Buy this Research Report (Insights, Charts, Tables, Figures and More) –https://www.maximizemarketresearch.com/checkout/3843/
From AI-Powered CTMS to Major CRO Mergers: The Moves Transforming the eClinical Solutions Market
On January 2024, Medidata Solutions launched an advanced Rave EDC module for decentralized trials, enabling remote data capture and digital-first study designs. On March 2024, Oracle Corporation introduced the upgraded Clinical One platform integrating EDC, CTMS, and RTSM with AI-driven analytics. On April 2021, Parexel was acquired by an EQT-led consortium to accelerate digital clinical services. On July 2021, PRA Health Sciences merged with ICON plc, forming a global CRO leader.
North America and Europe Lead the eClinical Solutions Market: AI, Cloud, and Decentralized Trials Driving Global Innovation
North America dominates adoption of AI-powered eClinical platforms and cloud-based clinical trial management solutions, controlling global trial design, accelerating decentralized trials, and setting the benchmark for digital clinical operations worldwide.
Europe leverages strict EMA regulations, GDPR compliance, and multi-country trial complexity to drive integrated digital clinical trial solutions, positioning itself as a hub for secure, patient-centric, and cross-border clinical innovation.
Both regions lead in cloud-based, AI-enabled eClinical software adoption, creating a competitive race in global clinical trial orchestration, real-world evidence integration, and next-generation decentralized trial technologies.
FAQs:
eClinical Solutions Market, Key Players
North America
1. Medidata Solutions (United States)
2. Oracle Corporation (United States)
3. Parexel International Corporation (United States)
4. PRA Health Sciences (United States)
5. Medrio Inc. (United States)
6. eClinicalWorks (United States)
7. IBM Corporation (United States)
8. Veeva Systems (United States)
9. Bio-Optronics Inc. (United States)
10. Clario (United States)
11. Datatrak International Inc. (United States)
Europe
12. ArisGlobal (Ireland)
13. ICON plc (Ireland)
14. CRF Health (Finland)
15. ERT (Germany)
16. Signant Health (United Kingdom)
17. Clinipace (Germany)
18. Anju Software Inc. (Germany)
Asia Pacific
19. eClinicalWorks India Pvt. Ltd. (India)
20. Tigermed (China)
21. CMIC Holdings Co., Ltd. (Japan)
22. Mediscience Planning Inc. (Japan)
Middle East and Africa
23. ClinTec International (United Arab Emirates)
24. Bioscript Group (South Africa)
South America
25. DATAMED (Brazil)
26. ClinTrial Refer (Brazil)
27. TrialStat Solutions Inc. (Brazil)
Explore the Full Market report – https://www.maximizemarketresearch.com/market-report/global-eclinical-solutions-market/3843/
FAQs:
1. What is the projected growth of the eClinical Solutions Market by 2032?
Ans: Global eClinical Solutions Market is projected to grow from USD 13.75 Billion in 2024 to nearly USD 35.76 Billion by 2032, expanding at a CAGR of 12.69%, driven by AI-powered clinical trial platforms, cloud-based solutions, and adoption of decentralized and patient-centric trial technologies.
2. Which product and segment are leading the eClinical Solutions Market?
Ans: Clinical Trial Management Systems (CTMS) dominate the market, serving as the operational backbone of global trials. Cloud-based and AI-enabled platforms are rapidly gaining traction, while EDC and eCOA solutions are increasingly integrated for real-time data capture, patient engagement, and regulatory compliance.
3. Which region dominates the eClinical Solutions Market?
Ans: North America leads the global eClinical Solutions Market, driven by high clinical trial activity, innovation-friendly regulations, early adoption of AI-powered platforms, and centralized control over trial design and data orchestration. Europe follows as the second-most dominant region, leveraging EMA regulations, GDPR compliance, and multi-country trial complexity.
Analyst Perspective:
From an analyst perspective, the eClinical Solutions sector is rapidly evolving, driven by AI-powered platforms, cloud-based trial management, and decentralized trial adoption. Competitive dynamics are intensifying as leading players like Medidata, Oracle, and Veeva focus on platform integration, strategic partnerships, and technology upgrades. Regional adoption in North America and Europe is shaping global trends, while emerging decentralized, patient-centric, and AI-enabled solutions present significant growth potential and strategic opportunities for sponsors and CROs worldwide.
Related Reports:
Preclinical CRO Market: https://www.maximizemarketresearch.com/market-report/global-preclinical-cro-market/109335/
Clinical Trials Market: https://www.maximizemarketresearch.com/market-report/clinical-trials-market/189646/
Clinical Trial Supply and Logistic Market: https://www.maximizemarketresearch.com/market-report/clinical-trial-supply-and-logistic-market/125037/
Clinical Communication & Collaboration Market: https://www.maximizemarketresearch.com/market-report/global-clinical-communication-collaboration-market/109896/
Clinical Diagnostics Market: https://www.maximizemarketresearch.com/market-report/global-clinical-diagnostics-market/116258/
Clinical Oncology Next Generation Sequencing Market: https://www.maximizemarketresearch.com/market-report/clinical-oncology-next-generation-sequencing-market/100383/
About Maximize Market Research – eClinical Solutions Market:
Maximize Market Research is a leading market research and business consulting firm, delivering actionable insights for the eClinical Solutions Market within the healthcare domain. Our data-driven research and strategic analysis support pharmaceutical companies, CROs, and healthcare organizations in navigating AI-powered platforms, cloud-based trial management, and decentralized clinical trial adoption.
Domain Focus – Healthcare for eClinical Solutions Market:
With a strong focus on healthcare and digital clinical innovations, Maximize Market Research helps clients identify growth opportunities, competitive dynamics, and emerging trends in eClinical Solutions. Our research empowers decision-makers to optimize clinical trial operations, enhance patient-centric solutions, and implement advanced technologies, positioning them for sustainable growth in a rapidly evolving global market.
Contact:
Lumawant Godage
MAXIMIZE MARKET RESEARCH PVT. LTD.
+91 96073 65656
Email: [email protected]
Visit Our Web Site: https://www.maximizemarketresearch.com/
LinkedIn.com: https://www.linkedin.com/company/maxmize-market-research-pvt-ltd/
Instagram: https://www.instagram.com/maximizemarketresearch/
Facebook: https://www.facebook.com/maximizemarketresearch/
X (Twitter): https://x.com/MMRAnalytics
Photo: https://mma.prnewswire.com/media/2891023/eClinical_Solutions_Market.jpg
Logo: https://mma.prnewswire.com/media/2457992/5781658/Maximize_Market_Research_Logo.jpg
View original content to download multimedia:https://www.prnewswire.com/news-releases/eclinical-solutions-market-to-surpass-usd-35-76-billion-by-2032–fueled-by-ai-driven-clinical-trial-management-solutions-as-analyzed-by-maximize-market-research-302684139.html
SOURCE Maximize Market Research Pvt. Ltd.