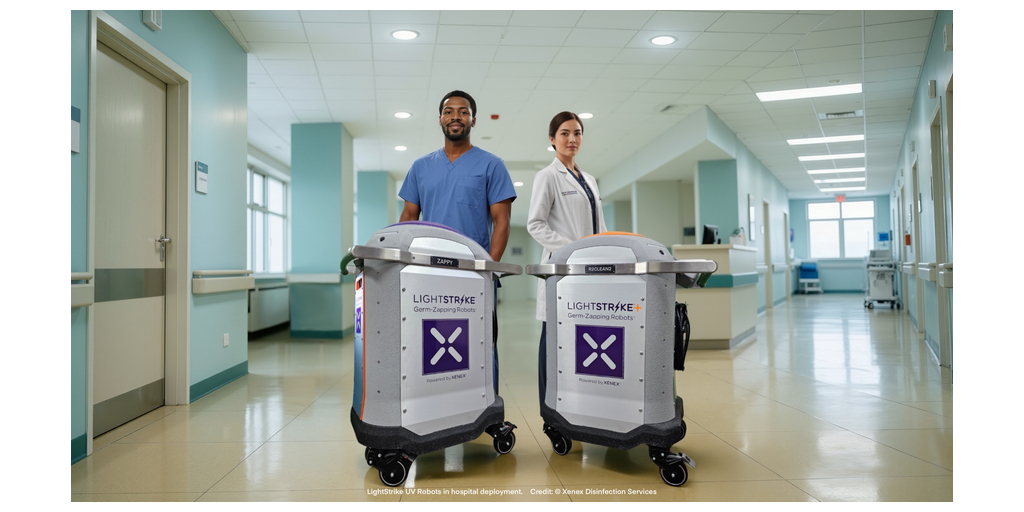
SAN ANTONIO, Texas–(BUSINESS WIRE)–Xenex Disinfection Services today announced that its LightStrike6™ (LS6) UV-C whole room sanitizing robot has received Registration from Health Canada for sale and distribution to healthcare facilities across Canada. The Health Canada Registration for LightStrike6 builds on the U.S. Food and Drug Administration (FDA) authorization of the LightStrike+™ as a no-touch “Whole Room Microbial Reduction” device. With the Health Canada Registration, Xenex becomes the first and only company with ultraviolet (UVC) room technology authorized in both Canada and the United States. Together, these authorizations create a new regulatory benchmark for validated, hospital grade UV room technology.
With antimicrobial resistance (AMR) accelerating at an alarming rate, the need for validated tools has never been greater. In the United States alone, antimicrobial-resistant infections cause millions of transmission events annually. Global and multilateral organizations—including the World Health Organization (WHO), Africa Centres for Disease Control and Prevention (Africa CDC), the Pan American Health Organization (PAHO), and the One Health Commission—have established commitments and action plans to combat antimicrobial resistance. Xenex will support healthcare facilities across Canada and beyond by providing an additional, science-backed tool to help reduce pathogen transmission and address the growing global threat of AMR.
LightStrike robots have been used in more than 1,200 facilities worldwide and are supported by 48+ peer reviewed studies showing significant reductions in microbial contamination and pathogen transmission on high-touch hospital surfaces when used after standard cleaning. LightStrike6 delivers the highest intensity broad spectrum UV light to sanitize patient rooms, operating rooms and other clinical areas to significantly reduce the number of pathogens that survive traditional chemical cleaning on high-touch surfaces such as bed rails, overbed tables, call buttons and bathroom fixtures.
LightStrike technology provides an additional evidence-based tool to support hospital whole room terminal disinfection programs, with a focus on persistent organisms such as C. difficile, MRSA, VRE, and other harmful pathogens.
As environmental services (EVS) staff face pressure to turn rooms quickly while managing staffing constraints, validated UV technology has become a critical component of a hospital’s infection prevention program. LightStrike6 was engineered to integrate into Canadian EVS and IPAC workflows with its ease of use, short room sanitizing times, and support for monitoring utilization and performance.
Important considerations when evaluating a UV system should include:
1. Whole room coverage
2. Minimal impact on room turnover time
3. Regulatory authorization and validation against common hospital pathogens.
With FDA authorization in the United States for LightStrike+ and Health Canada Registration for LightStrike6, Xenex is the only company with UV-C robots authorized for healthcare use across North America. Manufactured in San Antonio, TX, LightStrike+ and LightStrike6 robots are available. Infection prevention, EVS and procurement leaders can learn more or request a LightStrike demonstration at www.xenex.com.
Contacts
Kimberly Manganello
Xenex Disinfection Services
210.853.2875
[email protected]