BAD HERSFELD, Germany, Sept. 15, 2025 /PRNewswire/ — p36 GmbH, a leading provider of digital compliance solutions for the life sciences sector, today announces the official launch of UDI Connect, a next-generation SaaS solution purpose-built for medical device companies who’ve already established robust internal UDI governance – and are now ready to automate, integrate, and scale their compliance across global markets.
Bridging the Last Mile of UDI Compliance
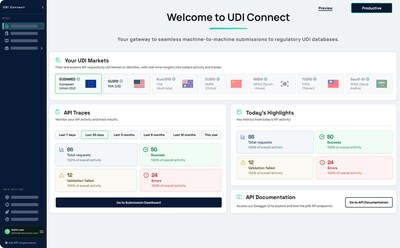
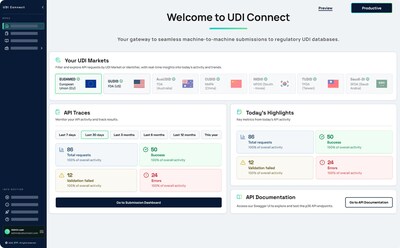
UDI Connect addresses a persistent gap in UDI processes: while many MedTech organizations have invested heavily in robust data validation and internal workflows, the final step, submitting data to regulatory databases such as EUDAMED, GUDID, and others, often remains manual, error-prone, and labor-intensive.
“Across the industry, strong internal systems were common, but manual submissions to regulatory authorities continued to create bottlenecks and compliance risks,” says Patrick Pfau, Co-Founder and Managing Director of p36. “UDI Connect bridges that gap. It brings true automation, real-time validation, and seamless integration into the IT landscape including SAP, PLM, and other systems, our clients have already built.“
Key Features:
- API-First Integration: Connects directly to existing systems (SAP, custom databases, etc.) – no rip-and-replace.
- Automated Validation & Submission: Instantly checks and submits UDI data to multiple global authorities, reducing manual effort and risk of human error.
- Real-Time Insights & Full Auditability: Comprehensive dashboards and audit trails provide full transparency, supporting both compliance teams and auditors.
- Scalability for New Markets: Enables organizations to expand their product portfolios globally and enter new markets – such as the EU and US – without requiring changes to existing internal workflows.
State of the Art: Compliance for MedTech
As regulatory deadlines like EUDAMED 2026 approach, UDI Connect empowers organizations to advance from semi-automated to fully automated, end-to-end compliance processes. Whether expanding into new markets or preparing for regulatory inspections, UDI Connect offers the missing link between internal governance and global regulatory requirements.
A Regulatory Affairs Manager at a leading MedTech company who participated in the pilot program stated: “Our old process was too manual and complex, especially with the EUDAMED deadline ahead. With UDI Connect, we’ve streamlined compliance and look forward to adding new markets more easily in the future.“
About p36 GmbH
Since 2019, p36 GmbH has been empowering world-leading life science companies across the globe with tailor-made cloud software. As an independent, owner-managed technology company, we specialize in consulting and developing SaaS solutions like UDI Connect that simplify regulatory processes, drive digital transformation, and help organizations stay compliant in an ever-evolving industry. Discover more about our commitment to innovation at: http://www.udihub.io
For further information:
|
p36 GmbH Michael Teufel Senior Marketing Manager
|
Photo – https://mma.prnewswire.com/media/2771825/P36_UDI_Connect.jpg
Logo – https://mma.prnewswire.com/media/2771824/5507463/P36_Logo.jpg
View original content to download multimedia:https://www.prnewswire.com/news-releases/p36-launches-udi-connect-automation-driven-udi-compliance-for-medtech-teams-whove-outgrown-manual-submissions-302555002.html
SOURCE P36