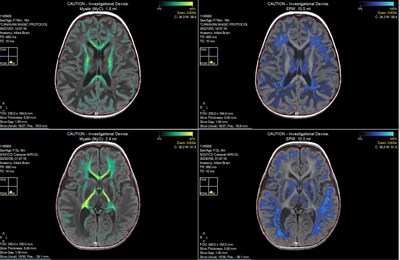
Groundbreaking study demonstrates that targeted oligodendrocyte gene therapy leads to decreased N-acetylaspartate (NAA) levels and increased brain myelin volume, resulting in promising functional improvements in children with Canavan Disease
NEW YORK, Sept. 16, 2025 /PRNewswire/ — Myrtelle Inc. (“Myrtelle” or the “Company), a pioneering clinical-stage gene therapy company developing transformative treatments for neurodegenerative diseases, today announced the publication of interim results from its Phase 1/2 clinical trial of rAAV-Olig001-ASPA (MYR-101) in Nature Medicine. The published article, entitled, “Oligodendrocyte-targeted adeno-associated virus gene therapy for Canavan disease in children: a phase 1/2 trial,” showcases a significant advancement in the treatment of Canavan disease, demonstrating promising outcomes from the single dose therapy in children affected by the disorder.
rAAV-Olig001-ASPA (MYR-101) is the first-ever recombinant AAV gene therapy designed to target oligodendrocytes, the brain cells responsible for producing myelin. The therapy directly addresses the enzymatic deficiency caused by mutations in the ASPA gene, which leads to impaired metabolism of N-acetylaspartate (NAA) and severe dysmyelination in Canavan disease.
“The reductions in CSF NAA levels, gains in myelin volume, and functional improvements in treated patients mark a compelling step forward for patients who currently have no approved treatment options,” said Adrian Stecyk, Chief Executive Officer of Myrtelle. “The Nature Medicine publication underscores the potential of rAAV-Olig001-ASPA to serve as a disease modifying therapy for Canavan disease”
About the Clinical Trial
Myrtelle’s First-in-Human (FIH) trial utilizes a proprietary recombinant AAV vector to deliver a functional ASPA gene directly to oligodendrocytes. In Canavan disease, these cells fail to produce the enzyme aspartoacylase, leading to disrupted myelination. The investigational therapy aims to restore enzyme activity, enable proper NAA metabolism, and facilitate myelin repair.
Key Results from the Study:
- Study presents interim results from the eight children with typical Canavan disease, followed for up to two years post-treatment.
- Preliminary results indicate that MYR-101 therapy is well-tolerated, with no serious adverse events attributed to the gene therapy itself.
- Significant reduction in NAA levels in cerebrospinal fluid (CSF) provides early evidence of therapeutic effect.
- Significant increases in brain myelin volume were demonstrated using Synthetic MRI (SyMRI), indicating new myelination.
- Participants showed developmental improvements versus historical controls, as assessed by the Mullen Scales of Early Learning (MSEL).
- All participants improved in at least two or more MSEL domains, with most improving in three or more, reflecting broad functional gains.
- Results support the potential of MYR-101 as a disease-modifying therapy, with ongoing follow-up assessing long-term outcomes and durability of response.
Regulatory Highlights
rAAV-Olig001-ASPA (MYR-101) was recently selected by the U.S. Food and Drug Administration (FDA) for inclusion in the Support for Clinical Trials Advancing Rare Disease Therapeutics (START) pilot program—one of only four CBER-regulated gene therapies to receive this distinction. The program provides enhanced regulatory support to expedite development of promising therapies for rare diseases.
The product has also received the following regulatory designations:
- Regenerative Medicine Advanced Therapy (RMAT)
- Orphan Drug, Rare Pediatric Disease, and Fast Track designations from the FDA
- Orphan Drug Designation and Advanced Therapy Medicinal Product (ATMP) classification from the European Medicines Agency (EMA)
- Innovative Licensing and Access Pathway (ILAP) designation from the UK Medicines and Healthcare products Regulatory Agency (MHRA)
About Myrtelle
Myrtelle Inc. is a gene therapy company focused on developing transformative treatments for neurodegenerative diseases. The Company has a proprietary platform, intellectual property, and portfolio of programs and technologies supporting innovative gene therapy approaches for neurodegenerative diseases. Myrtelle has an exclusive worldwide licensing agreement with Pfizer Inc. for its Canavan disease program. For more information, please visit the Company’s website at: www.myrtellegtx.com.
About Canavan Disease
Canavan disease (CD) is a fatal childhood genetic brain disease caused by mutations in the ASPA gene (ASPA) which prevent the normal expression of aspartoacylase, a critical enzyme produced in oligodendrocytes. The lack of normal aspartoacylase expression negatively impacts brain bioenergetics and development, including myelin production. Patients with CD are impacted at birth but may appear normal until several months old when symptoms begin to develop. Poor head control, abnormally large head size, difficulty in eye tracking, excessive irritability, severely diminished muscle tone, and delays in reaching motor milestones, such as rolling, sitting, and walking, are the typical initial manifestations of CD. As the disease progresses, seizures, spasticity, difficulties in swallowing, and overall muscle deterioration emerge with most affected children developing life-threatening complications by approximately 10 years of age. Currently, there are no cures for CD, and only palliative treatments are available.
More information on Myrtelle’s clinical trial in Canavan disease can be found on https://clinicaltrials.gov/ under the identifier NCT04833907 or by emailing [email protected].
Forward-Looking Statements
This press release contains forward-looking statements. Words such as “may,” “believe,” “will,” “expect,” “plan,” “anticipate,” “estimate,” “intend” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Forward-looking statements are based upon current estimates and assumptions and include statements regarding Myrtelle developing transformative treatments for neurodegenerative diseases. While Myrtelle believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based in information available to us on the date of this release. These forward-looking statements are subject to various risks and uncertainties, many of which are difficult to predict, that could cause actual results to differ materially from current expectations and assumptions from those set forth or implied by any forward-looking statements. Important factors that could cause actual results to differ materially from current expectations include, among others, Myrtelle’s ability to deliver the first approved therapy for Canavan disease; the program demonstrating safety and efficacy, as well as results that are consistent with prior results, the ability to generate the data needed for further development of this novel gene therapy in the patients with Canavan disease, and the ability to continue its trials and to complete them on time and achieve the desired results. All forward-looking statements are based on Myrtelle’s expectations and assumptions as of the date of this press release. Actual results may differ materially from these forward-looking statements. Except as required by law, Myrtelle expressly disclaims any responsibility to update any forward-looking statement contained herein, whether as a result of new information, future events or otherwise, our ability to pursue our regulatory strategy, our ability to advance ongoing partnering discussions, our ability to obtain regulatory approvals for commercialization of product candidates or to comply with ongoing regulatory requirements, our ability to develop strategic partnership opportunities and maintain collaborations, our ability to obtain or maintain the capital or grants necessary to fund our research and development activities, our ability to complete clinical trials on time and achieve desired results and benefits as expected, regulatory limitations relating to our ability to promote or commercialize our product candidates for specific indications, acceptance of our product candidates in the marketplace and the successful development.
View original content to download multimedia:https://www.prnewswire.com/news-releases/myrtelle-announces-nature-medicine-publication-of-interim-results-from-its-phase-12-clinical-trial-of-investigational-gene-therapy-raav-olig001-aspa-for-canavan-disease-302557585.html
SOURCE Myrtelle, Inc