In laboratories across the world, pH measurement is a fundamental process—whether you’re analyzing water quality, developing pharmaceuticals, or conducting chemical research. Even small variations in pH can significantly affect experimental results, chemical reactions, and biological processes. That’s why maintaining precision and reliability in pH measurements is not just desirable—it’s essential.
However, the accuracy of pH readings doesn’t depend solely on sophisticated instruments; it also relies on proper calibration, consistent maintenance, and the quality of the pH calibration buffer solution. Without these, even the most advanced pH meters can drift over time, leading to unreliable data and costly errors.
Let’s explore why calibration is vital, how buffer solutions work, and best practices to ensure long-term precision in pH measurements.
- Why Accurate pH Measurement Matters
pH—short for “potential of hydrogen”—is a measure of how acidic or basic a solution is. The scale ranges from 0 to 14:
- Below 7: acidic
- Above 7: basic (alkaline)
- At 7: neutral
In research and industry, maintaining the correct pH can determine the success or failure of a process. For example:
- In pharmaceuticals, incorrect pH can affect drug stability or reaction yields.
- In food and beverage production, it influences taste, safety, and shelf life.
- In environmental testing, pH helps assess pollution and water quality.
- In biotechnology, precise pH control ensures proper enzyme activity and cell growth.
Given its wide importance, laboratories depend heavily on accurate pH measurements—and that’s where calibration becomes crucial.
- The Role of Calibration in pH Measurement
Over time, pH meters and electrodes can experience drift due to electrode aging, contamination, or environmental factors. Calibration compensates for these changes by aligning the meter’s readings with known reference values from buffer solutions.
Calibration effectively ensures that when your meter reads “7.00,” it truly represents a neutral solution—and not a value skewed by electrode wear or sample residue.
Regular calibration not only improves accuracy but also helps detect issues like slow electrode response, faulty junctions, or contamination before they affect critical measurements.
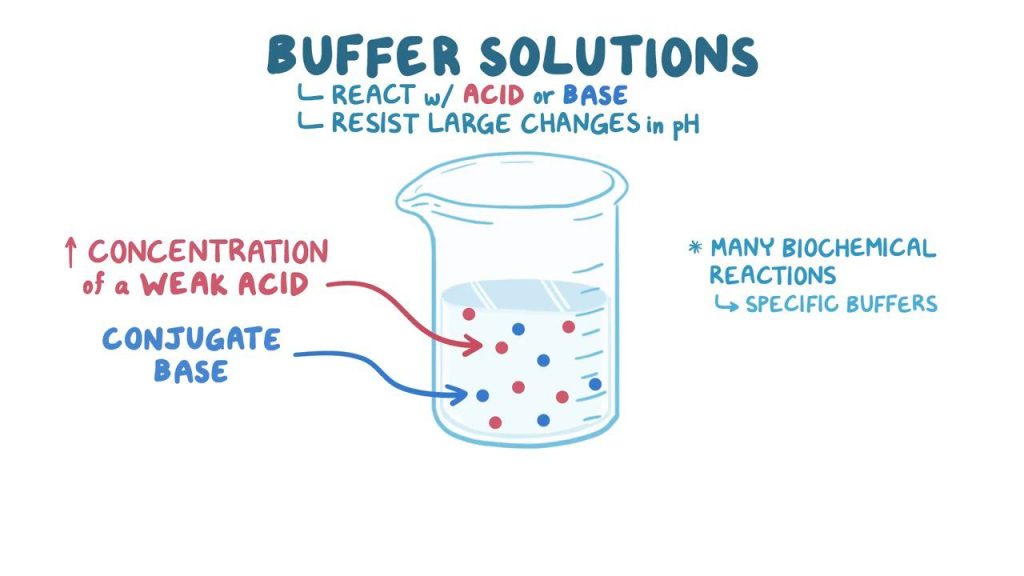
- Understanding pH Calibration Buffer Solutions
A pH calibration buffer solution is a specially formulated liquid with a precisely known and stable pH value. These buffers act as reference points for adjusting and verifying the accuracy of pH meters.
Common standard buffer values include:
- pH 4.00 (acidic buffer)
- pH 7.00 (neutral buffer)
- pH 10.00 (alkaline buffer)
These are typically chosen to bracket the expected pH range of the samples being measured. For example, if you’re analyzing slightly acidic solutions, you would calibrate using pH 4.00 and 7.00 buffers.
Buffers are formulated with chemicals that resist pH changes even when exposed to minor contamination or dilution—hence the term “buffered solution.” They maintain their stability under standard lab conditions, providing a reliable baseline for calibration.
- Types of pH Buffer Solutions
Not all buffers are created equal. Laboratories use different types based on their precision requirements and usage conditions.
- Standard (Prepared) Buffers
These are ready-to-use commercial solutions with traceable certification. They provide high accuracy and are ideal for laboratories that require consistent, reproducible results.
- Technical or Working Buffers
Used for routine calibrations where ultra-high precision is not essential. They are more cost-effective but may have a shorter shelf life or lower accuracy tolerance.
- Powdered Buffers
Sold as dry packets that can be dissolved in distilled water when needed. They’re useful for fieldwork or when storage conditions make liquid buffers impractical.
Regardless of type, certified buffer solutions should always reference NIST (National Institute of Standards and Technology) standards or equivalent traceability to ensure credibility.
- How to Calibrate a pH Meter Correctly
Proper calibration ensures your pH readings remain accurate and reliable. Here’s a step-by-step guide:
- Clean the Electrode: Rinse with distilled water to remove residues from previous measurements. Avoid wiping, as it can create static.
- Select the Correct Buffers: Use at least two buffer solutions that bracket your expected sample range (e.g., 4.00 and 7.00, or 7.00 and 10.00).
- Immerse the Electrode in the First Buffer: Wait for the reading to stabilize, then press the calibration button or manually set the meter to the buffer’s value.
- Rinse Between Buffers: Prevent cross-contamination by rinsing the electrode with distilled water between each buffer.
- Calibrate with the Second Buffer: Repeat the process to adjust the slope of the electrode’s response curve.
- Verify with a Third Buffer (Optional): For high-precision work, use a third point to ensure linearity across a wider pH range.
After calibration, test the electrode with a known solution to verify accuracy before proceeding with actual measurements.
- Best Practices for Maintaining Buffer Solutions
Even the best pH calibration buffer solution can give inaccurate results if not handled properly. Follow these guidelines to preserve accuracy:
- Avoid contamination: Never pour used buffer back into the bottle. Always pour a small amount into a separate container for use.
- Keep containers sealed: Exposure to air and CO₂ can alter buffer pH, especially for alkaline solutions (pH 10).
- Store at room temperature: Avoid direct sunlight or extreme heat.
- Check expiration dates: Old or discolored buffers should be discarded.
- Label clearly: Always mark opened bottles with the date of first use.
Regularly replacing your buffers ensures that your calibration is based on stable, trustworthy reference points.
- Common Sources of pH Measurement Error
Even with careful calibration, errors can still creep into pH readings. Common causes include:
- Electrode contamination: Residues from previous samples can affect the glass membrane’s sensitivity.
- Temperature fluctuations: pH readings vary with temperature—use automatic temperature compensation (ATC) if available.
- Dehydrated electrodes: Store electrodes in appropriate storage solutions, not distilled water, to maintain the glass bulb’s hydration layer.
- Incorrect buffer selection: Always use buffers that match your sample’s expected range.
By identifying and controlling these factors, you can maintain consistent precision in every measurement.
- The Role of Temperature Compensation
Temperature can significantly affect both the pH electrode’s response and the buffer’s actual pH value. Many modern pH meters include automatic temperature compensation (ATC), which adjusts readings based on the current temperature.
If your meter lacks ATC, manually note the temperature and refer to the correction tables provided with your buffer solutions. This ensures you’re reading the true pH value rather than one skewed by temperature changes.
- Quality Control and Documentation
For laboratories following strict quality assurance protocols (such as ISO 17025 or GLP compliance), proper documentation is essential. Always record:
- Calibration dates and times.
- Buffer lot numbers and expiration dates.
- Electrode ID and maintenance actions.
Routine verification against certified reference materials helps demonstrate traceability and ensures compliance with laboratory standards.
- Final Thoughts
Accurate pH measurement is a cornerstone of reliable laboratory science. It depends not just on advanced instruments, but on meticulous calibration, high-quality reference materials, and consistent maintenance practices.
The pH calibration buffer solution may seem like a small part of the process, but it’s the foundation on which accuracy is built. By using certified buffers, calibrating regularly, and handling solutions correctly, laboratories can ensure that every reading is consistent, reproducible, and scientifically sound.
In the end, precision in pH measurement is more than just a technical goal—it’s a commitment to quality, integrity, and the pursuit of reliable science.