Over the past five years, AI has supercharged drug discovery. Algorithms now routinely identify new compounds, predict protein folding, and simulate early-stage efficacy, all in silico. Billions of dollars have poured into AI drug startups like Insitro, Xaira, and Genesis, promising faster, cheaper, and more precise development of new therapies.
But while the lab bench has become digital, the bottleneck has simply shifted downstream to clinical trials.
Today, getting a promising drug candidate into the hands of patients still means navigating a $140 billion industry built on spreadsheets, PDFs, and manual back-and-forth. Identifying the right research sites, negotiating contracts, enrolling patients, and ensuring protocol compliance can take months or even years, costing up to $500,000 per patient. And despite this investment, more than half of clinical trials fail to meet enrollment targets.
That’s the broken reality Ryght AI is trying to fix.
From Protocol to Patient at AI Speed
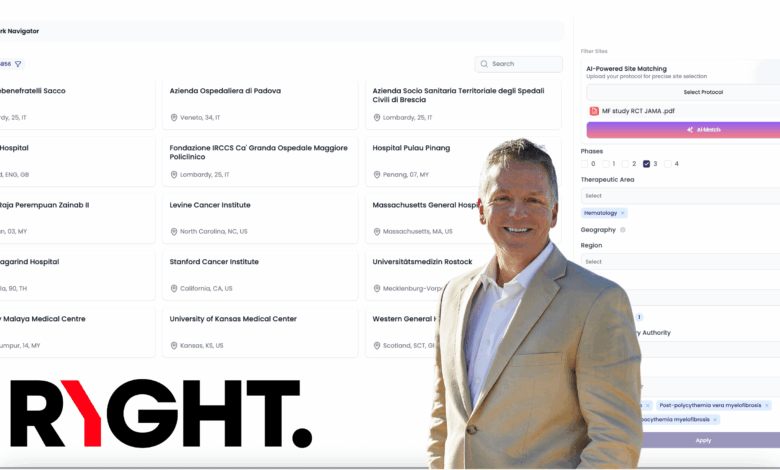
Ryght AI is building what it calls the world’s first “agentic AI platform” for clinical trials. The company’s system automates site selection, feasibility, budgeting, patient pre-screening, and even protocol authoring, areas that currently bog down timelines and strain sponsors, CROs, and research sites.
“We’re not just optimizing workflows, we’re replacing them,” says Simon Arkell, co-founder and CEO. “Ryght AI functions as an intelligent layer that connects trials with the sites and patients that can actually deliver.”
At the heart of the platform is a network of over 100,000 “digital twins” of trial sites worldwide. These AI-powered profiles continuously update based on public data, site-submitted information, trial registries, and live engagement data. When a sponsor uploads a protocol, Ryght’s agents parse the trial requirements, match them with qualified sites, and initiate automated outreach, feasibility checks, and even budget generation.
It’s a process that used to take months to years, now compressed into days and weeks.
Clinical Research Needs AI-Native Tools
Clinical trials have long struggled with inefficiencies. Sponsors still rely on outdated spreadsheets to evaluate sites. Feasibility studies often hinge on guesswork and slow surveys. And EMR integrations, while promising, are costly and slow.
Ryght takes a different approach. Rather than retrofitting AI into existing systems, the company has built a platform from the ground up, AI-native and focused on real-world usability.
“We learned from our experience with Deep Lens that much of pre-screening doesn’t happen in the EMR,” Arkell explains, referring to his previous company, which was acquired in 2022. “It happens face to face, when a coordinator or physician is with a patient. Ryght gives them AI tools they can use in real time, without waiting on integrations.”
The platform also includes built-in support for informed consent authoring, protocol simulations, site budgeting, and predictive analytics. These features not only speed up site activation but help trial sponsors anticipate problems, like slow enrollment or protocol misalignment, before they happen.
Strong Growth Early On
Since its founding in 2024, Ryght has raised over $9 million from investors including AIX Ventures and Foothill Ventures. The company has already signed nine U.S.-based academic medical centers, including Emory University, USC’s Keck School of Medicine, and the Medical College of Wisconsin. Several large CROs and biopharma sponsors have also adopted the platform, though many metrics are still under wraps.
What’s clear is the speed: Ryght can activate a new site in under a week. Compare that to the industry average of 3–6 months.
If Ryght delivers at scale, the implications are significant. Faster site activation means faster enrollment, which shortens time-to-market and reduces the cost per approved drug, savings that can reach into the hundreds of millions.
What’s Next on Ryght’s Roadmap
Ryght’s roadmap includes expanding global site onboarding, deepening its protocol simulation tools, and layering on predictive analytics to help sponsors mitigate trial risks earlier. The team is also focused on further automating budget negotiations, contract workflows, and document generation.
“Our vision is to unite the world’s clinical trial sites into one intelligent, real-time ecosystem,” says Arkell. “The faster we can run trials, the faster we can get life-saving drugs to patients.”
As AI continues to generate a wave of new therapeutics, Ryght hopes to ensure they’re not stuck in limbo, waiting on signatures, spreadsheets, and site delays.
Because when the AI drug pipeline fills up, it’s not discovery that breaks. It’s everything after.



