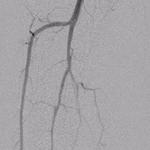
Injectable Angioplasty Balloon Catheter Transforms Peripheral Procedures, Users Report Reduced Equipment and Contrast Needs
First In-Man Experience Exceeds Expectations, Cited as “Game Changer” for Below-the-Knee Procedures CAMBRIDGE, Mass.–(BUSINESS WIRE)–Summa Therapeutics,... Read more.

Healthmine Unites Strategic Tech Vision and Consulting Practice
DALLAS–(BUSINESS WIRE)–As health insurers grapple with ongoing change and increasing market competition, Healthmine is renewing its commitment to offering... Read more.

Grant Thornton named one of Glassdoor’s Best Places to Work in 2024
The firm secures a spot on Glassdoor’s 16th annual Top 100 list CHICAGO–(BUSINESS WIRE)–Grant Thornton LLP, one of America’s largest providers of... Read more.

GoNetspeed to Soon Power Ludlow with 100% Fiber Internet Network, Construction Kicks Off
LUDLOW, Mass.–(BUSINESS WIRE)–GoNetspeed today announced that the Town of Ludlow will soon have access to 100% fiber internet with construction having... Read more.

Lido Advisors Partners With Constellation Wealth Capital to Further Accelerate M&A and Organic Growth
LOS ANGELES–(BUSINESS WIRE)–Lido Advisors (“Lido”), a leading registered investment advisor serving high-net-worth clients, today announced that... Read more.

Procyon Partners Brings on Harry Kirkpatrick as Chief Revenue Officer
With Over $6.5 Billion in Client Assets, the Juggernaut of RIA M&A and Top-Tier Advisor Recruiting Sets Sizeable Growth Goals for 2024 ST. PETERSBURG, Fla.–(BUSINESS... Read more.
Attune Medical® Announces Support of AF Symposium 2024
Company’s ensoETM® holds FDA marketing authorization for reducing the likelihood of ablation-related esophageal injury resulting from radiofrequency cardiac ablation... Read more.

JEDEC JC-14.7 Subcommittee Broadens Focus to Include Radio Frequency Technologies
Subcommittee Plans Meeting in April 2024 and Welcomes Industry Participation ARLINGTON, Va.–(BUSINESS WIRE)–JEDEC Solid State Technology Association,... Read more.

Airbyte and Vectara Partner to Simplify Data Access for Generative AI Applications
Data connectors for more than 350 sources facilitate building generative artificial intelligence applications SAN FRANCISCO–(BUSINESS WIRE)–#dataconnectors—Airbyte,... Read more.

Li-ion Battery Safety Trends and Impacts on Materials, 2023 Study – Igniting Growth, Fueling Innovation, and Paving the Way for New Opportunities – ResearchAndMarkets.com
DUBLIN–(BUSINESS WIRE)–The “Li-ion Battery Safety Trends and Impacts on Materials” report has been added to ResearchAndMarkets.com’s... Read more.
